Food Physics

- account_circleHironori HondohPhD, Assoc. Prof.
- account_circleKoki RyoPhD, Research Asst. Prof.
- Website:https://dfns.u-shizuoka-ken.ac.jp/labs/foodphys/
- Mail:hondoh@u-shizuoka-ken.ac.jp
- Phone:+81-54-264-5222
Physicochemical aspects in food structuring
Physics in Foods
- 1. Physicochemical aspects in food structuring
Structuring of foods can be considered as the self assembling process of components, crystallization, aggregation, and adsorption, based on the thermodynamics to minimize the free energy even if it is a non-equilibrium system. Egg will be hardened if it is heated, and jelly also will be hardened when it is cooled. We believe these processes can be understood with physical chemistry. The denaturation of proteins, partial crystallization of polysaccharides, which will be in continuous but different energy levels, makes it more difficult to understand their behavior. We are developing novel methods to investigate the structure of these condensed food systems.
- 2. Structural Changes in Foods
The structure of foods will be broken down by chewing, and can be changed during storage. Chocolate will be whitened when it is kept for a long period, or kept under a high temperature condition with the growth of needle-shape cocoa butter crystals. These processes are significant for the textual property and storage stability of foods. We are developing “in-situ” observation methods to understand these changes.
- 3. Crystal Growth of Biological Macromolecules
Highly ordered microscopic structure can be considered as a crystal. Fat can be crystallized when it is cooled to form a fine crystalline network. Chocolate, butter and margarine are typical foods containing fat crystals. Assembly of collagens also can be considered as a crystallization process since they form a well-ordered helical structure. Understanding of crystal growth of these materials is helpful to control the food structuring. We investigate fundamental processes with pure materials.
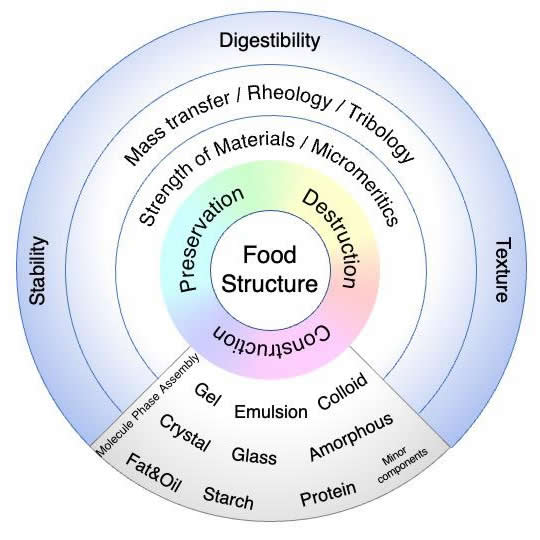
- Figure 1
- Wheel of Food Physics in Food Structure.
- Figure 2
- Structural change in food. Demulsification in a beverage.

References
- J. Am. Oil Chem. Soc., (2021) 98, 43-52.
- Molecules, (2020) 25, 5086-5099.
- J. Am. Oil Chem. Soc., (2020) 97, 1203-1213.
- Cryst. Growth Design, (2020) 20, 4980-4990.
- J. Am. Oil Chem. Soc., (2019) 96, 391-404.
- J. Am. Oil Chem. Soc., (2018) 95, 709-720.
- Cryst. Growth Design, (2018) 18, 4226–4229.
- Cryst. Growth & Design, (2017) 17, 6363-6371.