Molecular Reproductive Biology

- account_circleTohru KobayashiPhD, Prof.
- account_circleTaijyun MyosyoPhD, Research Asst. Prof.
- Website:https://db.u-shizuoka-ken.ac.jp/show/prof392.html
- Mail:tohruk@u-shizuoka-ken.ac.jp
- Phone:+81-54-264-5782
Studies examining the molecular mechanisms of germ cell differentiation and sex determination/differentiation
Cross-talk between germ cells and somatic cells during germ cell differentiation
It is also well known that environmental factors such as temperature and environmental endocrine disrupting chemicals affect the phenotypic sexes and reproductive phenotypes. We have also examined the molecular mechanisms of how environmental factors affect gonadal differentiation and development and reproduction. These studies are relevant to environmental preservation and may help improve aquaculture and fisheries.
- 1. Molecular mechanisms in the sex determination pathway
In the teleost fish medaka (Oryzias latipes), the sex determining gene dmy/dmrt1bY has been identified as the second sex-determining gene in vertebrates. We have examined the gene cascades in downstream pathway of dmy/dmrt1bY in detail.
- 2. Molecular mechanisms of sexual plasticity
In non-mammalian vertebrates, the sex reversal of gonads has been demonstrated in both sexes. In teleost fish, in particular, a complete sex reversal of gonads in both sexes can be induced by administration of sex steroid hormones, and in some cases, by modulating environmental factors. Using medaka and other model species such as tilapia and Xenopus, we have examined the molecular mechanisms of sexual plasticity in germ cells and gonadal somatic cells in detail.
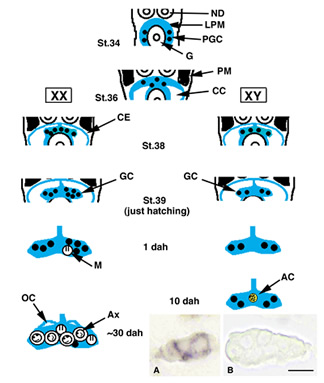
- Figure 1
- PM, paraxial mesoderm; LPM, lateral plate mesoderm; PGC, primordial germ cells; CE, coelomic epithelium; CC, coelomic cavity; ND, nephric duct; G, gut; M, meiotic cell; OC, ovarian cavity; AC, acinus structure; Ax, auxocyte. Inset, A sex determining gene, dmy/dmrt1bY expression by in situ hybridization. A, XY gonad at 0 dph. B, XX gonad at 0dph. dmy/dmrt1bY is expressed in Sertoli cell lineage cells of XY gonads specifically.
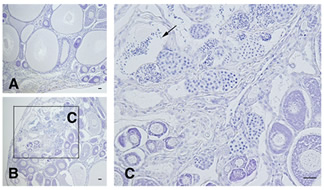
- Figure 2
- Mature female tilapia (XX) was treated by aromatase inhibitor (AI) for 4 months, resulting estrogen disruption. A, Control. B, C, AI treatment. A, Vitellogenic follicles were seen. B, Spermatogenesis was seen in a part of ovary. AI-treated fish finctioned as male. C, Large magnified in the area of section B. Arrow, Mature spermatozoa.
References
- Nature, 417, 559-563 (2002)
- Dev. Dyn., 231, 518-526 (2004)
- Proc. Natl. Acad. Sci. USA, 104, 3865-3870 (2007)
- Sex. Dev., 3, 108-117 (2009)
- Endocrinology, 151, 1331-1340 (2010)
- Int. J. Dev. Biol., 54, 105-111 (2010)