Green Chemistry
グリーンケミストリー-120x136.jpg)
- account_circleDaisuke NagaiPhD, Assoc. Prof.
- account_circleShusuke OkamotoPhD, Research Asst. Prof.
- Website:https://dfns.u-shizuoka-ken.ac.jp/labs/greenchem/
- Mail:daisukenagai@u-shizuoka-ken.ac.jp
- Phone:+81-54-264-5729
Development of Environmental Polymeric Materials Based on Well-Defined Polymer Synthesis
- 1. Development of a novel polymerization reaction
We develop novel controlled polymerization reactions. The controlled polymerization can be used to achieve a high degree of control over polymer chain architecture. Various type of polymers that can be synthesized include block copolymers, comb-shaped polymers, multiarmed polymers, and cyclic polymers. This control of structure results in polymers with widely diverse physical properties.
- 2. Synthesis of rare-metal recovery polymeric materials
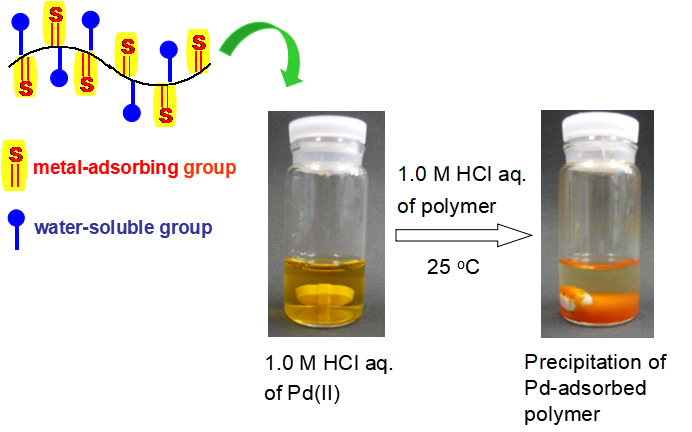
We developed a facile and high-recovery material for rare metals based on a polymer combining amino groups for water solubility and thiourea groups for metal complexation. Good solubility in aqueous metal ion solutions allows for homogeneous and efficient adsorption by the polymer, and a maximum PdII recovery amount (0.508 gPd/gpolymer) greater than those of other polymers reported in the literature (Figure 1).
- 3. Synthesis of organic-inorganic hybrid materials
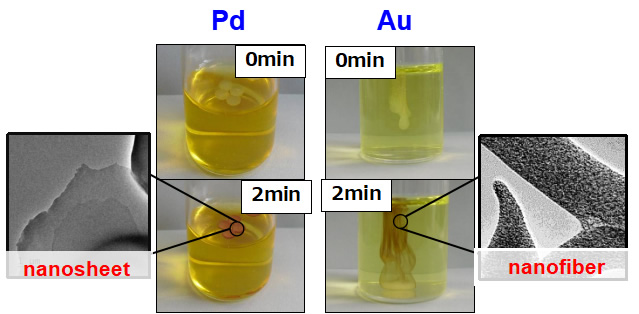
We examined self-assembled gelation behavior of a hydrophilic polymer bearing metal-coordination unit with metal ions (PdII or AuIII) upon addition of a dispersed aqueous solution of the polymer to an aqueous solution of metal ions. Self-assembled gels with different shapes were formed depending on the metal ions (Figure 2). Utilizing the mechanism for formation of different shaped gels, we succeeded in the synthesis of nanofiber containing uniformly dispersed Au nanoparticles and nanosheet containing PdII ions.
- Figure 1
- Photographs of water-soluble polymer with metal-coordination unit before and after PdII recovery (Chem. Commun., 2013).
- Figure 2
- Self-assembled gelation behavior of hydrophilic polymer with metal-coordination unit and metal ions (ACS Appl. Polym. Mater., 2020)
References
- ACS Appl. Polym. Mater., 2, 2211-2219 (2021)
- ACS OMEGA, 5, 18484-18489 (2020)
- J. Polym. Sci. Part A: Polym. Chem., 57, 2505-2510 (2019)
- Soft Matter, 14, 2712-2723 (2018)
- Sep. Pur. Technol., 177, 1-7 (2017)
- RSC Advances, 6, 103304-103310 (2016)
- RSC Advances, 5, 30133-30139 (2015)
- Chem. Commun., 49, 6852-6854 (2013)